Laboratory for the study of primordial germ cells
(Responsible: Prof. Massimo De Felici)
|
The laboratory is in the Section of Histology and Embryology of the Dep. of Biomedicine and Prevention, Faculty of Medicine and Surgery, University of Rome Tor Vergata.
From left to right: F.G Klinger, G. Rossi, M. De Felici, V. Rossi, S. Marcozzi, G. Salvatore e M. Sorrenti (2016)
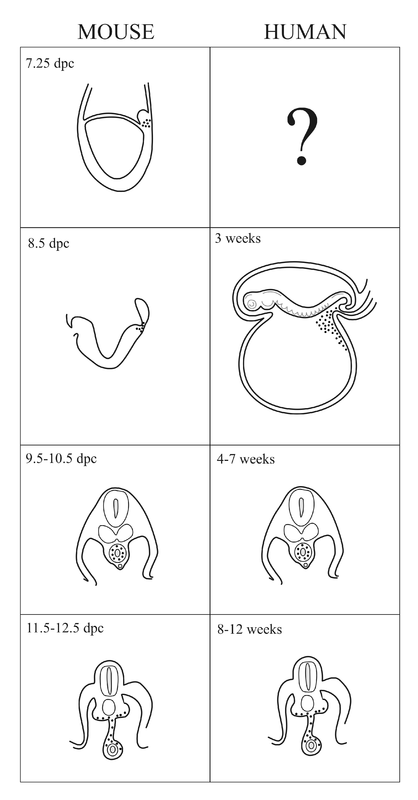
The life story of PGCs
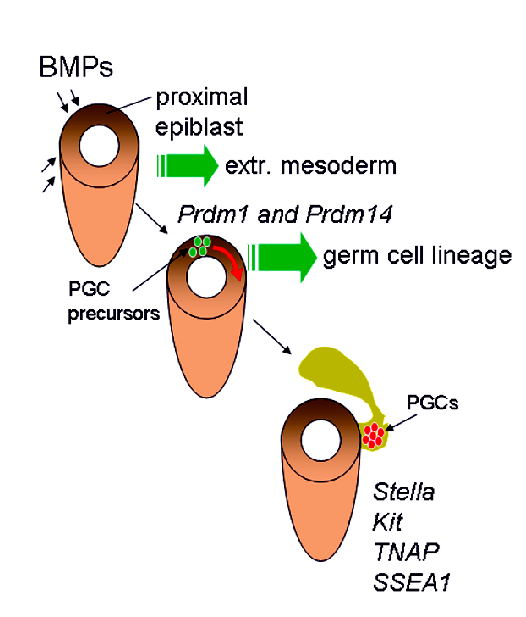
The main Lab research linesOver the last more than two decades the laboratory has contributed to develop methods for isolation, purification and culture mouse PGCs and to elucidate several aspects of PGC development in the mouse. Carrying out studies started in the early eighty years, at the Mammalian Developmental Unit in London under the guide of Anne McLaren, M. De Felici and his collaborators evidenced the crucial importance of certain cytokines such as LIF and SCF for preventing PGC apoptosis and sustain their survival. They also focused on characterizing adhesion molecules expressed in PGCs and performed the first studies aimed to elucidate their role in PGC migration. The search for compounds able to regulate PGC proliferation, led the laboratory to identify cAMP, retinoic acid and the PACAP neuropeptides as PGC mitogens. These findings served as base for studies that led P. Donovan’s and B. Hogan’s laboratories to the important discovery that PGCs can be transformed in culture in ES like cells termed EG cells. The regulation of the beginning of meiosis in female PGCs and the role of BMP4 in the formation of PGCs from the epiblast have been other topics to which lab researches have been devoted.
How PGCs arise?
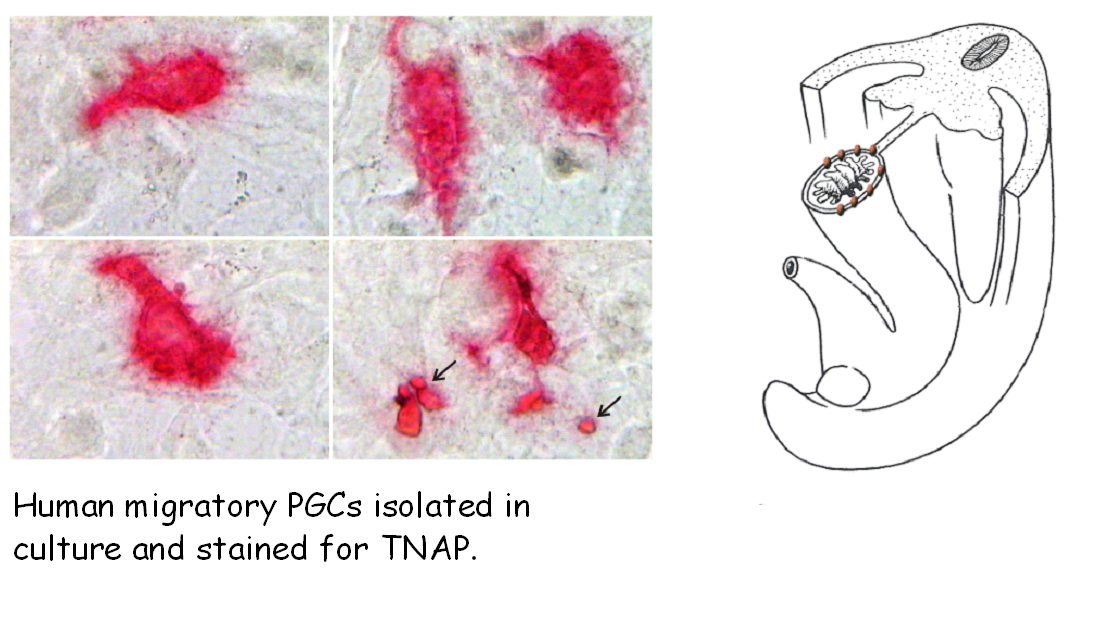
How PGCs migrate?
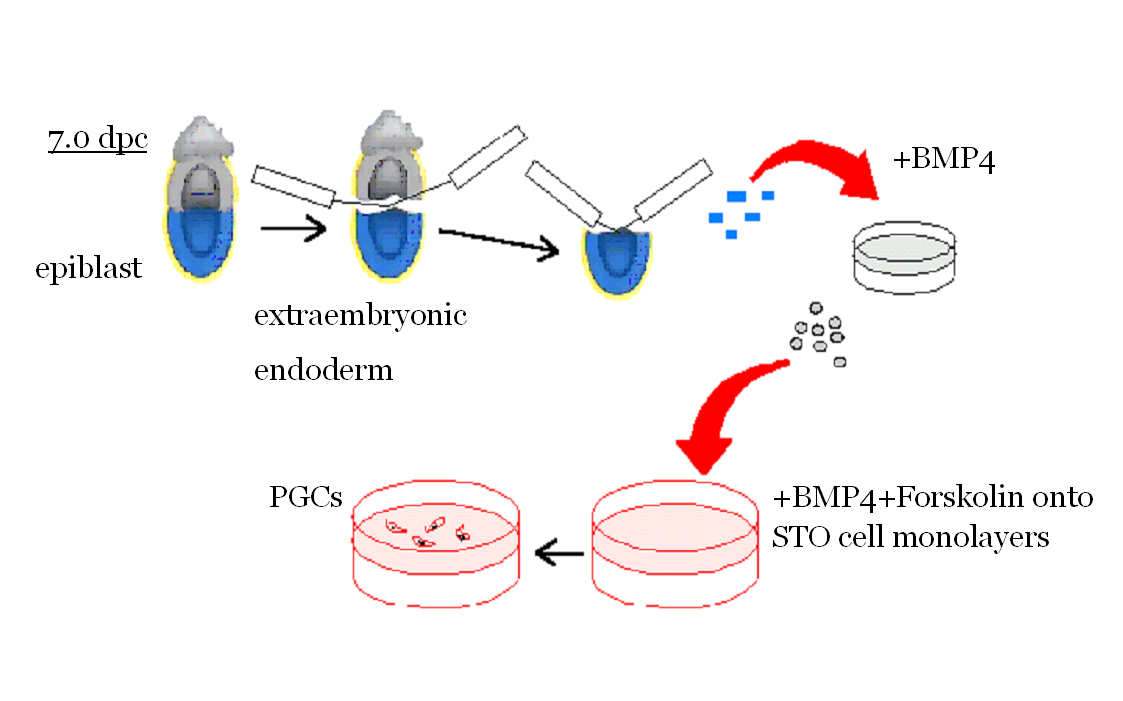
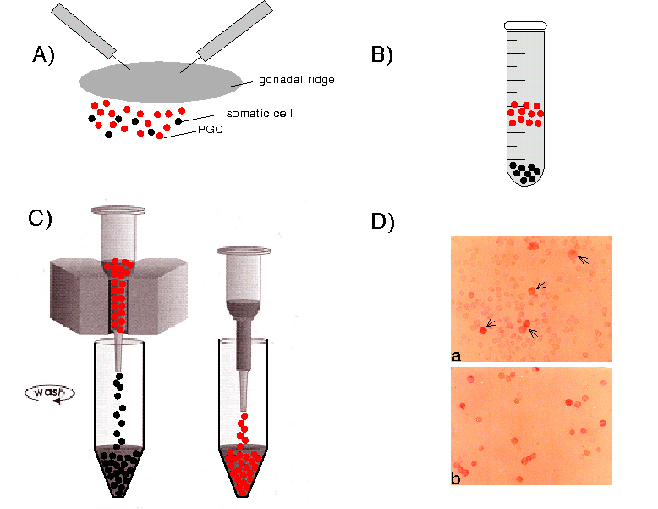
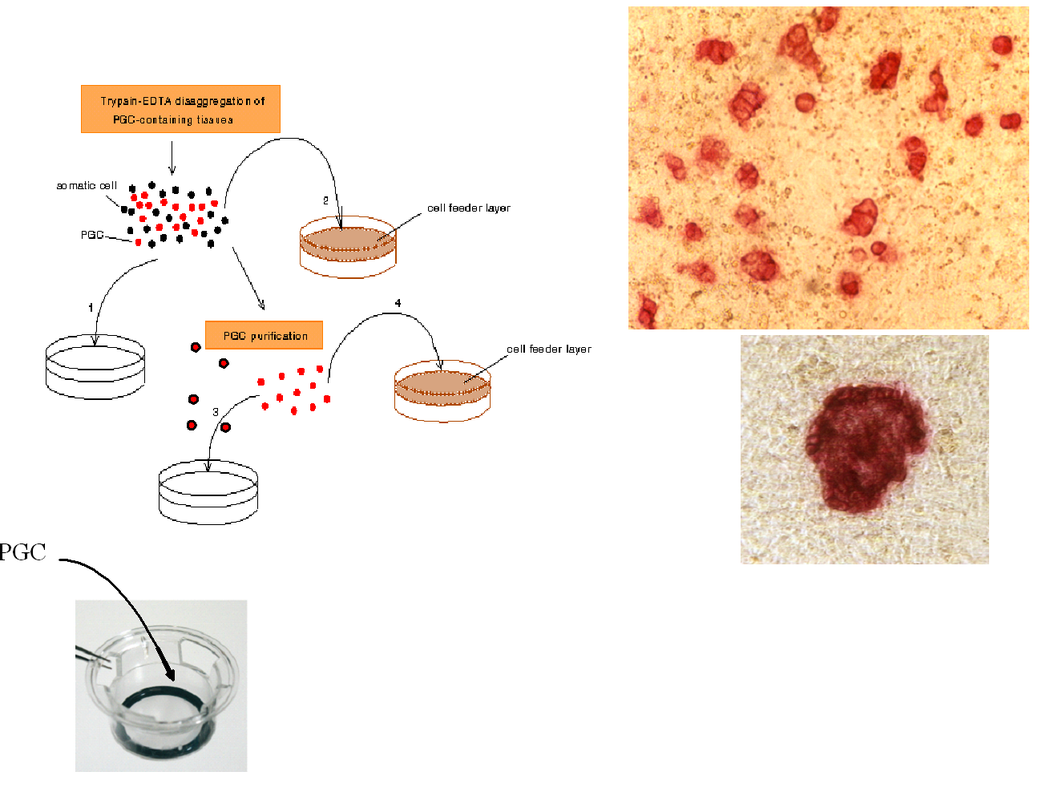
PGCs can be isolated and purified from the mouse embryo
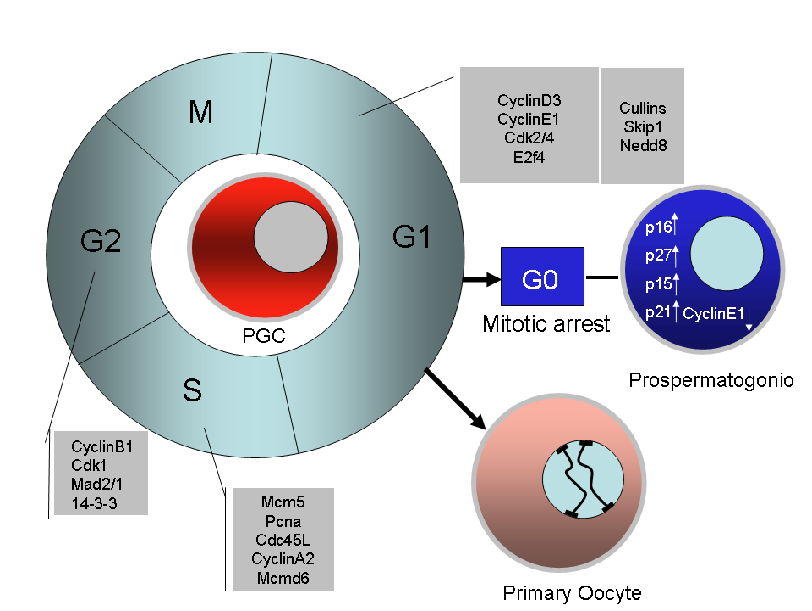
PGC proliferation
| |||||||||||||||||||||||
|
A diagram showing the main cell cycle genes found to be expressed and likely to control cell cycle in proliferating mouse PGCs (data are based on the papers of Sorrentino et al., 2007, Western et al., 2008 and some our unpublished observations.
|
Our studies have shown that cAMP or cAMP agonists such as forskolin (FRSK) markedly stimulate PGC proliferation. Molecules that may stimulate in vivo the intracellular cAMP increase in PGCs, have not been identified yet. We have found, however, that, among many peptides known to increase intracellular cAMP via specific receptors, pituitary adenylyl cyclase activating peptides (PACAPs) are able to stimulate in vitro proliferation of PGCs. In addition, we reported evidence about the presence of these peptides in the fetal gonads. Another compound that showed to be able to significantly increase the proliferation rate of PGCs was retinoic acid (RA). RA added to the culture medium in the range of 1-5 µM, promoted PGC proliferation on feeder layers of both their own gonadal somatic cells and of TM4 or STO cells.
|
Anti-apoptotic survival growth factors for mouse PGCs
|
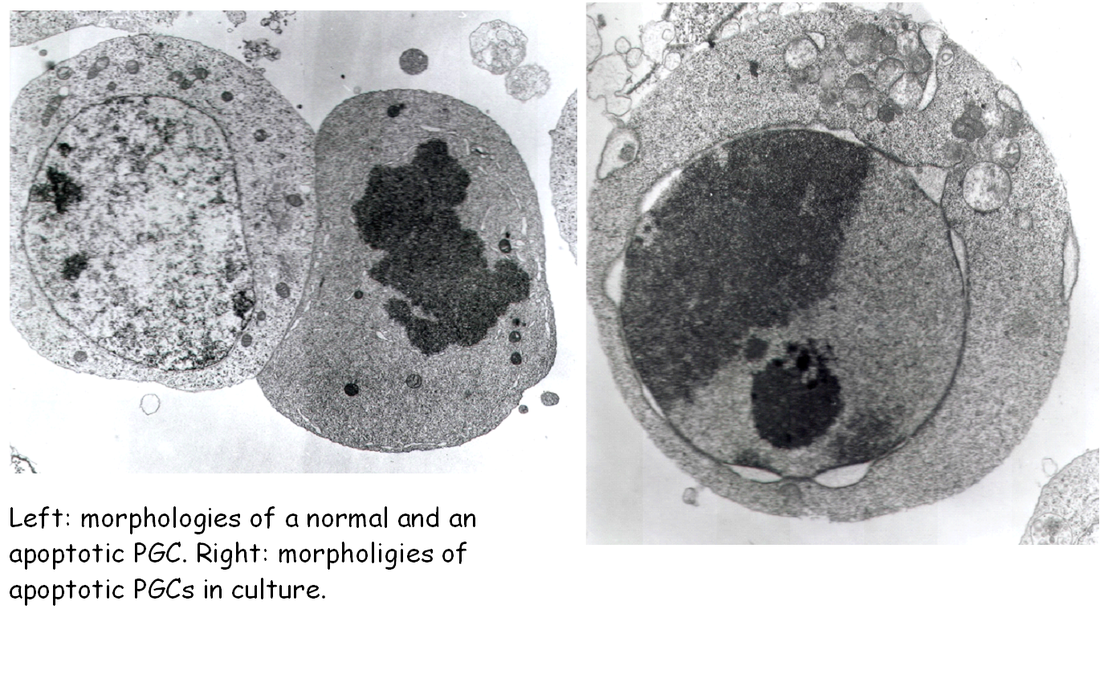
Thanks mainly to the use of two in vitro culture systems, PGCs co-cultured on feeder layers of their own gonadal somatic cells or of established cell lines, survival and/or proliferation factors needed for PGC development were discovered. The leukemia inhibitory factor (LIF) was the first growth factor identified in our lab as able to significantly increase PGC survival in vitro. A second growth factor found to exert a very important action on PGCs was the stem cell factor (Kl or SCF). In 1991, Susanna Dolci, working with the P. Donovan’s group at Frederick, showed, simultaneously with other laboratories, that SCF is essential for PGC growth in vitro, and that the PGC life-supporting activity of certain feeder cells is partly due to the production of such growth factor, in particular of the SCF membrane-bound form. In our lab, we apply for the first time the notion of the apoptotic degeneration to PGCs and found that the addition of SCF or LIF to the culture medium markedly reduced apoptosis in PGCs during the first hours of culture. More recently, we have shown that the effect of SCF on PGC apoptosis is, at least in part, due to a reduction of the expression of the pro-apoptotic gene bax. The finding that isolated PGCs begin to undergo apoptosis a few hours after isolation from the gonadal ridges, gave finally an explanation of their rapid degeneration in culture.
|
MPS/MIS?
|
It is well known that in mammals oocytes begin meiosis during fetal or early postnatal life in the ovary, while in male the first germ cells which enter leptotene stage are normally first seen in the young pre-puberal testis. Byskov (1974) suggested that a diffusible factor produced by the fetal rete ovarii (the meiosis-inducing substance, MIS) is involved in stimulating meiosis in the female. The reason why meiosis does not occur in male fetal germ cells has been explained by the action of a meiosis preventing substance (MPS) produced within the seminiferous cords (Byskov and Saxen, 1976; Byskov, 1978). Other observations favor the view that germ cells are intrinsically determined to enter meiosis unless they are prevented from doing so by being enclosed within the seminiferous cords of the testis. In 1990, De Felici and Dolci (Development 109, 37-40) approached the problem by producing in vitro chimeric mouse fetal gonads. Whereas most of donor 12.5 dpc female germ cells appeared able to enter meiosis within host ovarian tissues only a small number were in meiosis in chimeric testes. None or very few of donor male germ cells entered meiosis in ovarian host tissues. Aggregation of meiotic 13.5 dpc female germ cells with testis tissues from 13.5 to 14.5 dpc embryos resulted in inhibition of meiotic progression and pyknosis in most donor germ cells. These results support the existence of a meiosis preventing substance or a factor causing oocyte degeneration in the fetal mouse testis, but not of a meiosis inducing substance in the fetal ovary. More recent results, however, supported the early Byskov evidence indentifying retinoic acid as the MIS produced by the rete ovarii.
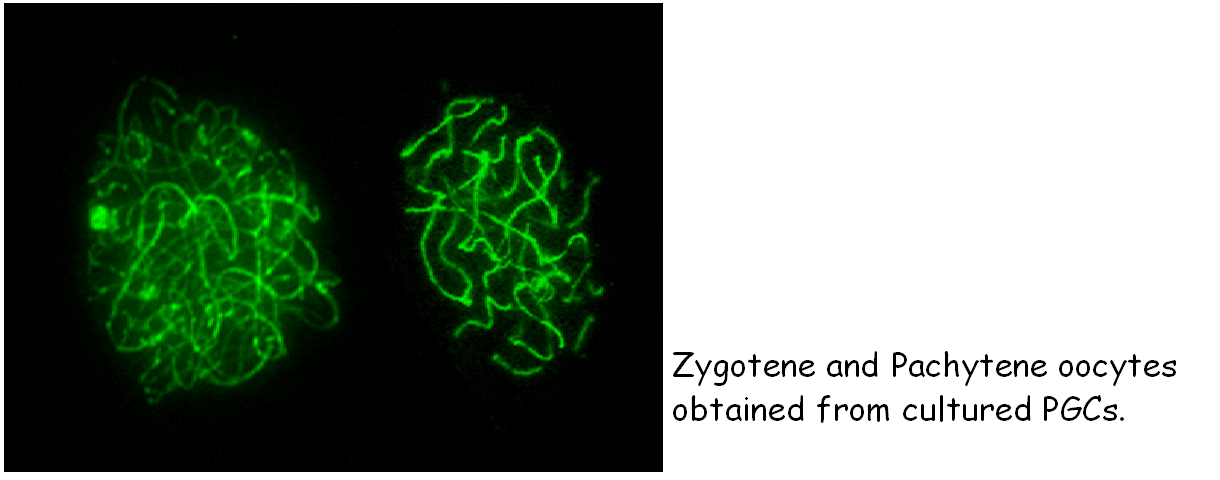
Using the culture method for isolated PGCs recently devised in the lab (Farini et., 2007, Dev Biol 306, 572-583), we hope to contribute to clarify some of such controversies. In particolar, we are now studing a protein called STRA8 believed to play a crucial role in the beginning of meiosis. |
The surprising behaviour of PGCs in vitro
|
De Felici and McLaren in pioneristic studies observed that germ cells isolated from embryonic gonads of different ages have different abilities to survive in vitro. The use of feeder layer culture, certain growth factors and compounds gradually led to improvements in PGC culture and to establish conditions that allowed PGCs not only to survive in vitro for several days, but also to proliferate. Several groups found that a combination of compounds (Forskolin, RA) and growth factors (SCF, LIF, bFGF), in certain culture condition, may lead to prolonged proliferation of a small population of PGCs and their transformation into totipotent ES-like cells, named embryonic germ cells (EG cells). A crucial enzyme involved in PGC trasformation in EG cells seems to be PTEN, a lipid phosphatase able to reduce the level of the phospholipid Pip3. development of embryos obtained from transplanted EG or EG cell nuclei. We have devised a new method and culture conditions that allows for the first time to culture PGCs in the absence of somatic cells to enter and progress into meiosis. We hope that this will allow understanding the mechanisms governing the crucial switch between mitosis and meiosis (Farini et al., Dev. Biol., 2007).
|
| references.pdf |
Staff
Group Leader: prof. Massimo De Felici
Collaborators: Dr. Donatella Farini, Dr. Francesca Gioia Klinger,
Post-doc: Valerio Rossi,
PhD students: Serena Marcozzi, Giulia Salvatore, Maria Sorrenti
Technicians: Gabriele Rossi
Collaborators: Dr. Donatella Farini, Dr. Francesca Gioia Klinger,
Post-doc: Valerio Rossi,
PhD students: Serena Marcozzi, Giulia Salvatore, Maria Sorrenti
Technicians: Gabriele Rossi
Indirizzo
Dipartimento di Biomedicina e Prevenzione
Facoltà di Medicina e Chirurgia
Università degli Studi di Roma “Tor Vergata”
Via Montpellier, 1
00133 Roma (Italy)
Facoltà di Medicina e Chirurgia
Università degli Studi di Roma “Tor Vergata”
Via Montpellier, 1
00133 Roma (Italy)
Contatti e-mail:
| staff.pdf |
| phd_and_phdc_in_the_lab.pdf |